About SORAPEX & Team
A short history about the people behind SORAPEX and how it came to existance.
Beate Sonntag
Beate is the founder and CEO of SORAPEX and provides services to the company’s clients.
She started her career with the decision to study pharmacy at the „Carolo Wilhelmina“ Technische Universität Braunschweig for a degree in pharmacy and the state examination as an apothecary. Whilst studying at one of the most reputable universities in Germany, she was an avid reader of all kinds of printed materials and played successfully volleyball up to state level.
Her past-time acitivities made her a very quick reader and allowed her to transfer the experiences in team sport to team work. She had to learn to be very organized to manage all her extra-curricular acitivities together with the very demanding workload at the university. As a consequence, Beate is able to efficiently prioritize workload and to work focused on details whilst dividing the sheeps from the goats.
Inspired by a start in regulatory affairs at a multinational pharmaceutical company in Germany shortly after her degree, Beate successfully added a diploma in European regulatory affairs from the universities of Leiden and Amsterdam in The Netherlands to her credentials. Given the profound basis of a degree in pharmacy, her approbation as an apothecary, and an advanced academic qualification in regulatory affairs she developed her profession in ascending multiple European and global regulatory affairs positions and projects during the following 20+ years, while specialising in the rather unpopular field of labeling.
Based on proven experience as a senior executive within regulatory affairs in the pharmaceutical industry, excellent project and people management skills and profound communication capabilities, Beate eventually decided create SORAPEX to offer her extensive knowledge and experience in regulatory affairs to the industry as an experienced expert, leader, manager, and trainer in all functions related to labeling.
Rainer Hübert
Rainer supports SORAPEX with administrative, marketing and IT tasks.
After his trade college degree in 1976 he joined the German Air Force which he left after his contract ended 8 years later as a First Lieutenant. The next few years he was searching for a fullfilling future career with first starting an apprenticeship at an renowned industrial photographer in Düsseldorf, Germany. A year later Rainer registered at Universität Duisburg majoring in political science with minors in philosophy and history. Another two years later he found his calling.
1989 Rainer started a management consultancy with a friend and provided advice to SMEs mainly in the fields of marketing, general business administration, and document management. A few years later he specialized in business process design and re-engineering, before he became an internationally renowned consultant, speaker and author on the topic of business continuity management.
In 2016 cancer threw Rainer off the tracks and forced him to end his career for good. In 2019 he accepted the offer of his friend Beate to assist her in running her business SORAPEX GmbH.
SORAPEX
Profile
In 2012 German pharmacist Beate Sonntag moved with her family from Hannover, Germany to Richmond upon Thames, United Kingdom. After arriving and settling the wish to engage in meaningul work and the new inspiring environment of Greater London led to the decision to create and establish SORAPEX Ltd. in England. Soon, SORAPEX Ltd. was successfully providing professional services for global regulatory affairs to the pharmaceutical industry.
The relocation back to Hannover, Germany in 2016 (early BREXIT, kind of) led to the business decision of dissolving the company in the UK and to continue the business operating as a professional freelancer (Freiberufler) from Germany. However, except for the location and the legal framework, neither the business model or services of the company nor its client base did change. The name and domain „SORAPEX“ remained in use, the description “Regulatory Affairs Services for Pharmaceuticals” was complemented, the latter being changed to „The Labeling Professionals“ in 2020 to better emphasise the core strengths and USP of SORAPEX.
Because of its success and growing client base, in 2019 the legal framework for the business was changed again and the company is now incorporated and officially registered in Germany as SORAPEX GmbH, with Beate Sonntag as its founder, owner and CEO. Also in 2019, SORAPEX hired the first employee, to relief Beate from administrative tasks and allow her to better concentrate on the demands of her clients.
Name
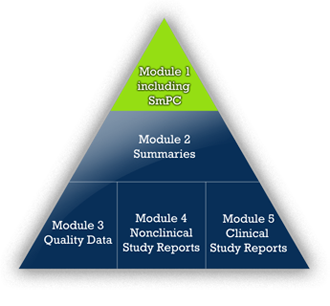
The idea for the company’s name derives from APEX. Originally Latin, the term APEX is known in German, English, and French. It describes the localization of the labelling information in the top triangle of the ICH Common Technical Document (CTD), Module 1.3. The SmPC is the „ultimate“ summary of the CTD.
Presenting the individual motivation of the founder and her focus on regulatory affairs, SORAPEX communicates the message of SOnntag Regulatory Affairs Pharmaceutical EXcellence.
Slogan
SORAPEX‚ slogan „The Labeling Professionals“ is aiming to summarize the high importance of labeling, especially within the Summary of Product Characteristics (SmPC) in the context of the well-known phrase „always read the label“, as used during the promotion of a product:
- The SmPC is the basis of information for health care professionals on how to use the medicinal product safely and effectively
- The SmPC is the key labeling regulation for the establishment of the patient information leaflet informing patients about the correct use of the medicinal product.
- The SmPC is one of the main documents in the public domain.
- The SmPC is published in national compendia in the corresponding national language (e.g. Vidal, Arzneimittel-Kompendium and eMC).
- The SmPC is the agreed position between the marketing authorization holder and the regulatory authority.
- The SmPC is the basis for any promotional message and is key to reimbursement.
- Proper labeling of products regulated by the FDA is extremely crucial. Improper labelling could cost a company millions of dollars in litigation and bad publicity.
SME Privileges
SORAPEX GmbH is a small sized enterprise (SME) according to the EU recommendation 2003/361 of the European Commission in combination with the Commission Regulation (EC) No 2049/2005. For our clients within the European Union, which are complying with the SME category of the regulations mentioned, we can offer significantly reduced registration and admission fees.
